The worldwide animal protein industry has a host of hefty challenges ahead. Producers are tasked with feeding a growing population while making the best management choices for their operations. Nutritionists and veterinarians are responsible for providing critical recommendations and consultation to their customers. Animal health companies, like Elanco, must continue to develop new technologies and solutions to propel the industry forward and meet the needs of everyone involved.
“Elanco is committed to innovation — and exploring existing technologies — to best understand our customers’ needs and develop the tools the industry needs to continue to succeed,” said K. Douglas Miller, M.S., Ph.D., Elanco Director of Global Beef Product Development and Brazil Research and Development (R&D) Operations. “Our customers are continually seeking new ways to use our products in their feedyard businesses. Having input from the product user level is key to our research and development team’s understanding of what needs we must meet and what innovations are yet to be discovered.”
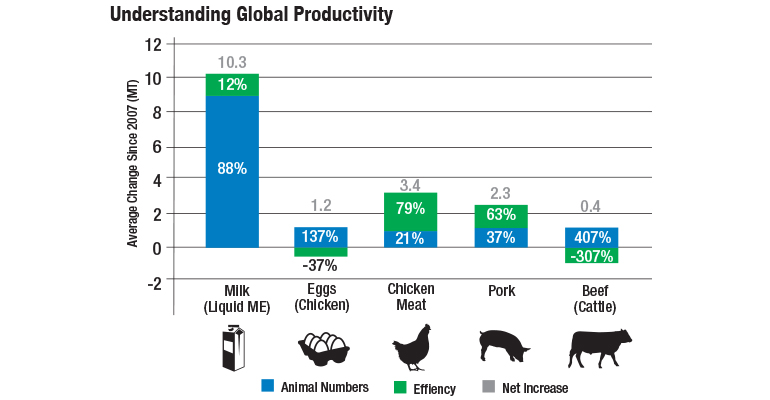
Understanding the trends of global protein productivity drives Elanco researchers to pursue new technologies that will enable producers to meet protein demand. The table below details global productivity trends that Elanco has tracked since 2007. This type of information helps Elanco understand the impact of providing sustainable solutions for improving product efficiency and keeping increases in animal numbers as minimal as possible.
The blue columns represent the percentage of animals the protein industry has added in order to meet demand, while the green columns represent more efficient practices and/or technologies implemented to help meet demand. Two species — eggs and beef — are trending backwards, which means animals have been added, without an increase in efficiency of production. Overall, global productivity is going backwards in an unsustainable manner. This trend — coupled with the ever-changing demand — emphasizes the importance of new technology development.
“The customer feedback cycle is critical to the success of our products over time.” – K. Douglas Miller, M.S., Ph.D.
Identifying solutions to fit today’s market
The team of Elanco experts is working diligently to keep technologies available to maximize producer success. Keeping products in alignment with regulations — as well as recognizing additional potential within the existing Elanco portfolio — is no small task for the Elanco R&D team. From modifying product label text within the United States Food and Drug Administration process to adding new product claims, the scientists are responsible for ensuring that Elanco’s products are optimized to benefit the industry. Using a wide range of industry contacts — from feedyards to universities — greatly assists Elanco in these efforts.
Miller said, “A cycle of feedback keeps the information flowing from our customer to our commercial group, and then back to our R&D team. This new knowledge drives the exploration of either a new use or a product evolution to create value for our producers. The customer feedback cycle is critical to the success of our products over time — we must have a balance of new innovation and incremental innovation.”
Senior Research Scientist Sandra Gruber, Ph.D., echoed the importance of seeking multiple sources of information to drive innovation.
“Advisory boards composed of diverse individuals such as nutritionists, veterinarians and university professionals represent varied industry viewpoints and provide some of the most valuable insights we receive,” Gruber said. “These advisory boards come to us with questions that challenge our R&D team, such as, ‘Is there space for us to optimize a current product to fit how these producers need to be able to use our products?’ The groups truly help us look at solutions for the real-world that could be applied both in the United States and on a global level.”
Seeking industry expertise for post-commercialization success
Cross-functional Elanco teams meet on a regular basis to discuss the potential of opportunities for additional product explorations. New developments can take the form of either a molecular extension or a product line extension. A molecular extension would involve moving an existing product or active pharmaceutical ingredient (API) into a new category — such as a new species.
This process can take approximately four to five years and requires an average $20 million investment.* A product line extension — such as adding a new indication to a product — takes about three to four years and is approximately a $10 million investment.*
“When we think about post-approval expansion of our product opportunities, it really is to make sure our customers realize the full potential of our APIs to best suit their needs. Some companies don’t prioritize innovation, much less post-approval product expansion and optimization,” Gruber emphasized.
Miller added, “There is a huge difference in the marketplace between the companies who provide services and value beyond product and those who do not. Elanco is proud to be counted as one of the pioneers moving the innovation path forward.”
Key points
- Elanco is committed to delivering innovation to the beef industry to drive progress
- Collection of industry insights propels development of real-world solutions
- The multi-year, multi-million-dollar innovation process delivers new opportunities for producer success
Hitting an innovation homerun
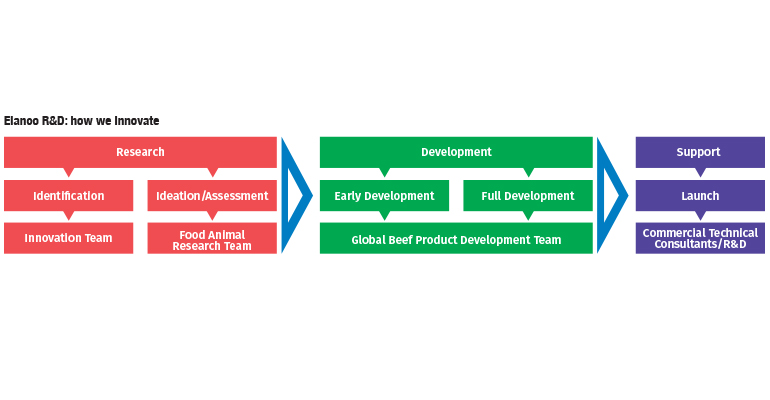
When promising new discoveries are made, Elanco researchers are involved at all levels, every step of the way. Discovery of a new molecular entity — a brand-new to market product — requires a development time frame of six to eight years (on average) and an investment of approximately $30 million.* According to Miller, the price tag varies depending on what species the product might be optimized for, and what potential is visible at the beginning development stages.
“In the early identification and ideation stages of research, we typically see about nine out of ten products fail,” said Miller. “The investment is fairly low at this point since we have so many projects occurring at this level. If a project seems promising, it gets elevated into the early development stage. Formulation science, safety assessments, duration and dose definition and manufacturing queries are all addressed at this point to truly understand the full-scale potential of this product. Our standards are very high, so only three out of ten projects make it past this early development into the full development stage.”
The full development stage involves a deep dive into the pivotal definitive studies of the potential product — as well as a major investment in the promising technology. Studies focusing on efficacy, human safety, target animal safety, manufacturing quality and environmental toxicology are all completed at this level and are communicated to the appropriate governing bodies to secure product registration. Technologies that make it to this stage of development have greater odds of success — approximately seven out of ten projects get the green light to proceed further. Moving forward depends on securing regulatory approval as well as continuing commercial viability assessments from Elanco product development teams. The next phase — launch and commercialization — only accepts the most elite and promising products. In total only about 2 projects out of 100 meet the high standards imposed and make it from ideation to customer utilization.
“Our producer customers see the products that make it to the marketplace — but not the discoveries that don’t make it the whole way,” Gruber noted. “Elanco’s efforts to keep the market aware of our constant work is ongoing. The length and challenge of the product development process — combined with regulatory requirements — present a monumental challenge for us to make sure we bring the best possible products to the marketplace. Elanco is proud to innovate today for the success of our industry tomorrow.”
To learn more about ongoing Elanco innovation, contact your Elanco sales representative or technical consultant, and visit Elanco.us for additional information.
*This average does not include the cost of employing the scientific expertise and personnel
required to support and lead the project.
Elanco and the diagonal bar logo are trademarks of Eli Lilly and Company or its affiliates.
©2017 Eli Lilly and Company or its affiliates.
fyfeed 5372-3
USBBUNON02140



Leave A Comment